Abstract
Introduction Ruxolitinib was the first Janus Kinase inhibitor (JAKi) approved for treatment of patients with myelofibrosis. The pivotal COMFORT-I and -II phase III trials demonstrated considerable efficacy in terms of reducing splenic volume and symptom burden, but also identified a possible signal for increased risk of non-melanoma skin cancers (NMSC). A subsequent analysis of the WHO pharmacovigilance database confirmed an association between ruxolitinib use and NMSCs1. However, very little data exists on the nature of NMSCs associated with ruxolitinib, their clinical outcomes and impact on patient management. We set out to investigate these parameters in a real-world analysis of UK MPN patients treated with ruxolitinib who have been diagnosed with NMSCs.
Methods We performed a retrospective data collection from 15 UK centres coordinated via the UK National Cancer Research Institute (NCRI) MPN working party. All patients receiving ruxolitinib for MPN and diagnosed with NMSC between 2010 and 2022 were eligible. Baseline MPN characteristics, skin cancer characteristics and outcomes were collated. The first NMSC diagnosis was defined as the primary event and subsequent episodes of the same cancer type were considered reoccurrences.
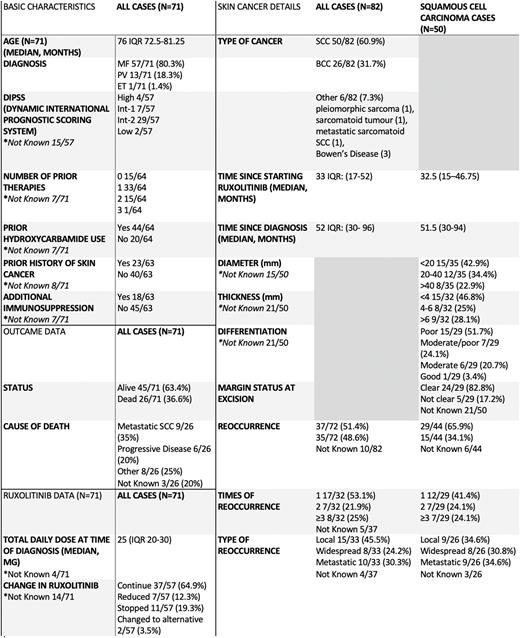
Results We identified 71 patients with a median age of 76 diagnosed with NMSC whilst receiving ruxolitinib: 50 squamous cell carcinomas (SCC), 26 basal cell carcinomas (BCC) and 6 other NMSC. The majority of patients had either underlying myelofibrosis or polycythaemia vera (80.3% and 18.3%, respectively) and 76.6% had at least one prior line of MPN therapy, including hydroxycarbamide in 68.7% of cases. A history of skin cancer predating the start of ruxolitinib was noted in 36.5%, and 28.6% had additional immunosuppression. The median total daily Ruxolitinib dose was 25mg.
NMSC occurred at a median of 52 months (IQR: 30-96) and 33 (IQR: 17-52) following diagnosis with an MPN and commencing ruxolitinib, respectively. Where dermato-pathological details were available for patients with SCC, most tumours had poor or moderate-poor differentiation (22/29). The treatment of choice was local excision with clear excision margins achieved in 24/29 of the cases. In 29/44 cases, SCCs reoccurred, with 14/29 reoccurring more than once. In 9/29 cases, recurrence was metastatic in comparison to 8/29 and 9/29 cases of widespread and local recurrence respectively. Following the diagnosis of a NMSC, ruxolitinib therapy was continued in 64.9% of cases. At the time of analysis, 26 patients had died, including 9 who died of metastatic SCC (34.6%) and 6 of progressive MPN (23.1%) .
Conclusion We hereby report on 71 ruxolitinib-treated MPN patients diagnosed with NMSC, representing the largest series described to date. The skin cancers demonstrated aggressive features, and high rates of recurrence, metastatic spread and mortality, similar to NMSCs seen in other heavily immunosuppressed cohorts2. It remains unclear whether this is purely due to the immunosuppressive properties of ruxolitinib or whether other factors e.g. prior hydroxycarbamide, older age and immune dysregulation may contribute. Most patients continued ruxolitinib after a diagnosis of NMSC, presumably reflecting good MPN control and lack of current evidence that cessation or switching to an alternative JAKi improves overall skin cancer outcomes.
Our study is limited by its retrospective nature which may have introduced a selection bias for more aggressive cases. Nonetheless, our data suggest that NMSCs diagnosed in MPN patients on ruxolitinib appear relatively aggressive. Patients should be counselled appropriately, especially those with high-risk features, including advice to minimise skin cancer risk and to report new skin lesions early, which should prompt rapid dermatological review and follow up.
Prospective studies are required to better characterise the risk of developing NMSC and their outcomes associated with all JAKi's and other potential novel agents. Lastly, collaborative work is required with a view to developing guidance on managing both the NMSC and the underlying MPN for this group of patients.
1. Jalles C et al. Skin cancers under Janus kinase inhibitors: A World Health Organization drug safety database analysis. Therapie. 2022 May PMID: 35710462.
2. Berg D et al. Skin cancer in organ transplant recipients: epidemiology, pathogenesis and management. J Am Acad Dermatol 2002; 47: pp. 1-17.
Disclosures
Rampotas:Gilead: Other: Conference Fees. Somervaille:Abbvie: Consultancy; Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Imago Biosciences: Research Funding; Oryzon Genomics: Consultancy; CellCentric Ltd: Research Funding. Psaila:Alethiomics: Consultancy, Current equity holder in private company, Honoraria, Other: Co-founder , Research Funding; Novartis: Consultancy, Honoraria, Speakers Bureau; Constellation Therapeutics: Consultancy; Blueprint Therapeutics: Consultancy; Galecto: Research Funding; Evotec: Research Funding. Mead:Alethiomics Ltd: Consultancy, Current equity holder in private company, Other: Co-founder and equity holder, Research Funding; Gilead: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Galecto: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Sensyn: Consultancy, Speakers Bureau; Karyopharm: Consultancy, Speakers Bureau; Sierra Oncology: Consultancy, Speakers Bureau; CTI: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau. Garg:Janssen, Takeda, Navartis, Amgen, BMS, GSK: Consultancy, Honoraria; Janssen, Amgen, Takeda, Novartis: Consultancy, Other: Ad Board; Janssen, Amgen: Consultancy, Speakers Bureau. Butt:Novartis: Honoraria, Speakers Bureau. Innes:Incyte: Speakers Bureau. Harrison:Constellation Pharmaceuticals, Inc., a MorphoSys Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; EHA: Other: Leadership role; Geron: Membership on an entity's Board of Directors or advisory committees; Sierra: Honoraria; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; AOP Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Galacteo: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Promedior: Membership on an entity's Board of Directors or advisory committees; MPN voice: Other: Leadership role; Gilead: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding. Godfrey:Novartis: Other: Personal fees; Celegene: Other: Personal fees; AOP Orphan: Other: Personal fees. McLornan:JAZZ: Honoraria, Speakers Bureau; CELGENE BMS: Research Funding, Speakers Bureau; ABBVIE: Speakers Bureau; NOVARTIS: Honoraria, Research Funding, Speakers Bureau. Lambert:Kite: Consultancy, Honoraria; Takeda: Other: Travel and conference fees; Bristol Myers Squibb: Other: Travel and conference fees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal